Varithena Varicose Vein Treatment
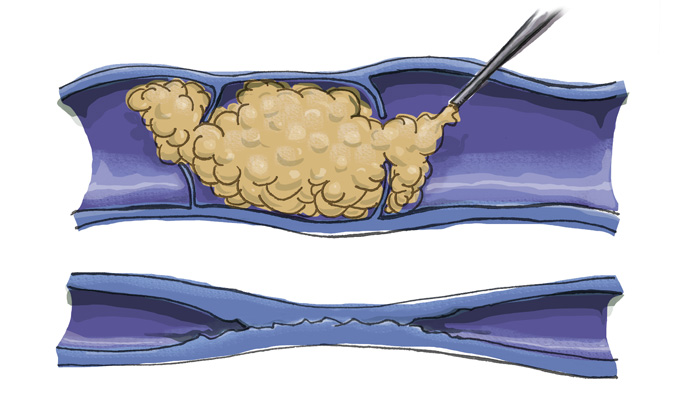
Nisha Bunke2020-12-19T04:24:50-08:00At La Jolla Vein Care, we are the experts in using foam sclerotherapy to treat varicose veins: we have performed thousands of successful treatments over a decade. We are pleased to offer Varithena foam. Varithena is the only FDA-approved foam treatment used to treat varicose veins caused by problems with the greater saphenous vein and other related veins in the leg’s greater saphenous vein system. Varithena is not thermal ablation and does not require multiple injections to numb the treatment site as with tumescent anesthesia. The administration of Varithena is minimally invasive: There are no incisions, sedation, or general anesthesia required. Patients can even go back to doing some activities the very same day as treatment, and the entire procedure should take less than an hour. Varithena® is a name brand of foam, that comes in a pre-made canister. Varithena® treats a wide range of varicose veins in the GSV system, including:
o Tortuous (twisted) veins
o Veins above and below the knee
o Veins with small, medium, and large diameters
o Veins previously treated with other methods.